(This is part one in a three-part series on integrating packaging machinery with track and trace serialization systems to meet the 2017 pharmaceutical mandates.)
Introduction
Pharmaceutical manufacturers have many good reasons to implement track and trace technology within manufacturing and packaging processes. Serialization allows manufacturers to ensure the integrity of their product and compliance with emerging pedigree laws. Designed primarily as a response to the increase of counterfeit pharmaceuticals, pedigree and e-pedigree laws (for electronic documentation) require manufacturers to show the complete life cycle of the drugs they distribute, from the manufacturing process through the end-of-line packaging.
 |
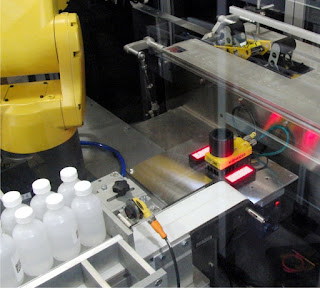
| 2D and Serialization Codes Used in Track & Trace Packaging Lines |
Unit level tracking methods have been in place for years. Lot/Expiration codes are ubiquitous on a wide variety of products. But recent changes to the law have shifted the focus to implementing track and trace systems with case packers and palletizers. This can present a number of challenges to pharmaceutical manufacturers, so selecting a qualified packaging machinery supplier to work with the track and trace system supplier is vital. Pharmaceutical companies already invest heavily in capital equipment for manufacturing and packaging. By integrating track and trace serialization technology with automated end-of-line packaging machinery, pharmaceutical manufacturers can meet pedigree requirements and maintain their levels of productivity in a single robust solution.
Packaging Machinery for Track & Trace Lines
Serialization systems track the product from the time it is
placed in its primary package (bottle, vial, tube, jar, pouch, etc.) to its
final placement on a pallet at the end of the packaging line. This requires
integrating inspection and tracking equipment from a track and trace system
supplier with the packaging machinery at each step in the packaging process. It’s
important to select an equipment provider who understands the requirements
being faced by pharmaceutical manufacturers. Ideally, the packaging machinery
supplier is also an integrator who can work with other OEMs to successfully
implement a track and trace packaging line.
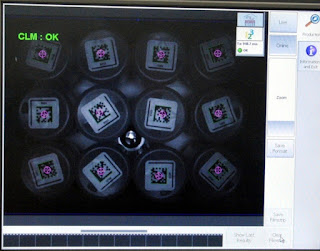 |
| Cameras for Scanning Product Codes (Top) and the Stored Code Information (Bottom) |
The track and trace systems incorporate several types of equipment.
First labeling or other types of coding equipment place a unique ID code on the
item being packaged, e.g. bottles, cartons, blister packs, bundles, etc. Next
cameras and other sensors that are capable of reading the ID are integrated
with the packaging machinery at various stages in the packaging process such as
cartoning or case packing. Print and apply labelers are integrated to label the
case with information about its contents, and these labels are also inspected
for accuracy. Reject systems are integrated to allow improper product to be
removed from the production stream.
The packaging equipment to be integrated with the serialization
system should also be considered carefully. Automated packaging machinery is
better able to handle production speeds needed to factor in the time it takes
to record the serialization information without diminishing overall production
rates. The process typically begins with the primary packaging equipment.
Individual products, be it bottles, cartons, trays, or bundles, are labeled
with a unique identifying code during the primary packaging of the product. It
is this code that the track and trace system uses to create information about
the contents of each carton, case and pallet.
Storing Serialization Data
Track and trace serialization systems incorporate a means for recording
and storing the serialization data for each production run. This is usually
accomplished through a dedicated PC integrated with the printers, cameras, and
sensors. The centralized data point allows the system to distribute serial
number information to each packaging level at each tracking point such as when
the product is cartoned, when the carton is case packed and when the case is
palletized. These systems interface with the packaging machinery controls to
allow the exchange of information.
 |
| PC Interface for Recording Serialization Information |
Next Post: Cartoners and Case Packers for Track and Trace Serialization